VACCINES are promising, but they can hardly be regarded as the cure-all for COVID-19 virus.
IMMUNE ESCAPE due to gain of function by the evolving SARS-COV-2 virus strain is the main challenge
Building an arsenal of multiple drugs that work in different ways is important, because COVID-19 is a multifaceted illness that affects different people in different ways
There will never be a silver bullet, a one-size-fits-all situation to address this infection
Clinical studies in COVID-19, large percentage of them have been designed in a way that it’s hard to learn from them.

The team at MR Khaitan Pharmatech Pvt Ltd (MRKPPL) has invented and filed patents in the US with a priority date of 05/05/2020 for a cure for Coronavirus (including drug delivery system)

Expected to be effective for all patients – Asymptomatic, mild symptoms (90% of total infected population), those suffering from ARDS due to COVID-19 infection

Invention based on sound scientific rationale and principles, hitherto not obvious

Freedom to operate analysis of the patent assures commercialization of drug therapy without fear of infringement

MRKPPL’s IND administered to patients suffering from SARS-Cov2 infection with ARDS in a hospital in Pennsylvania, displayed remarkable recovery and were taken off intubated oxygen within 3 days and discharged with minimal side-effects.
The drugs and the device are USFDA approved as individual ligands/device, and qualify for Phase 2 clinical trials.
Patients in India infected with the Delta variant also took the prophylactic therapy (on compassionate basis) without any specialized medical supervision and experienced speedy recovery with noticeable relief.
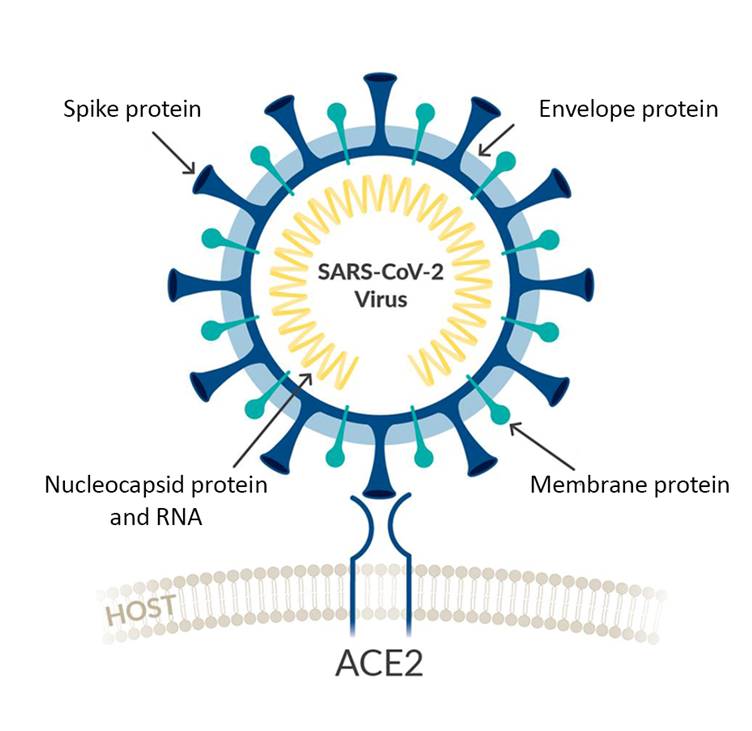
Coronaviruses are enveloped positive-stranded RNA viruses that replicate in the host cell cytoplasm. To release their nucleocapsid into the cell, they rely on the binding to the cellular receptors, angiotensin converting enzyme 2 (ACE2) for SARS-CoV-2, by major conformational changes of the virus spike glycoprotein (S) and entry into the cells by endocytosis.
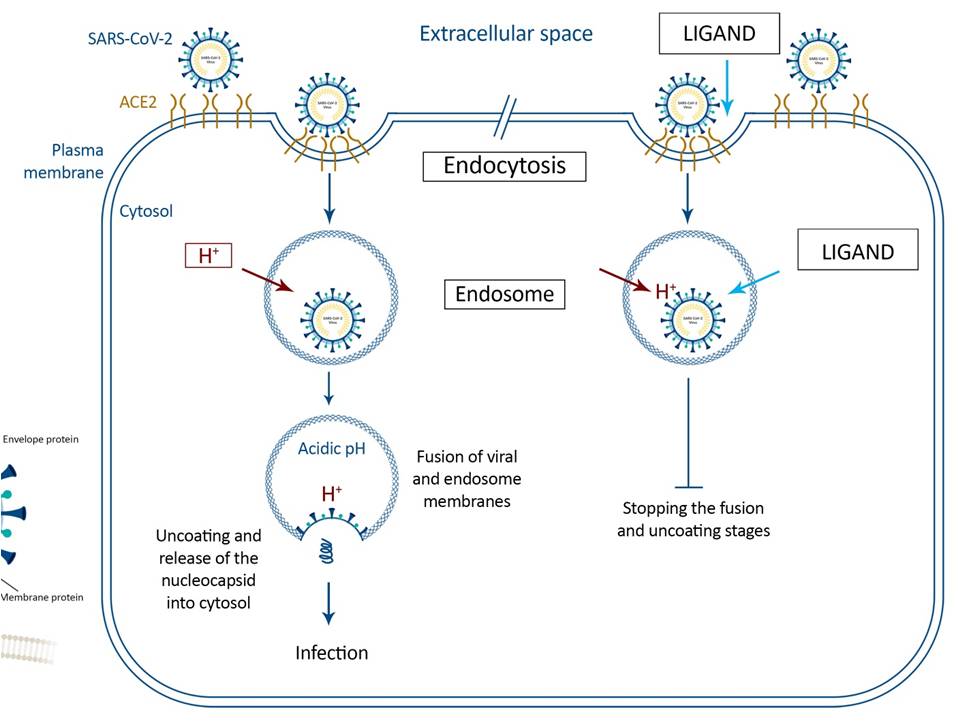
Pathway of endocytosis of SARS-CoV-2 into the target cells with Ligand (on the right) and without it (on the left). Binding of the virus S-protein to ACE2 promotes internalization into endosomes, where the optimal pH environment triggers fusion activity in the S-protein. Inhibitors of SARS-COV-2 replication using Δ pH to perturb the Proton Motive Force can be targeted to dissect the pathway because they specifically block pH-dependent membrane fusion by sharp reduction in half-life of the virus and aggregation of the S-spikes.
NOTE: The Ligand is the patent applied for drug which has shown highly encouraging results in case studies on patients suffering ARDS due to COVID-19 infection.
The first drug when inhaled into the lungs is converted by local esterases to its active metabolite, with a significantly higher glucocorticoid receptor binding affinity and anti-inflammatory activity. The purpose of this drug inhalation is to make the subject experience significant increase in Forced expiratory volume (FEV) and thus more receptive to the inhalation of the anti-viral drug which may cause irritation of the trachea. Significantly, isolated case studies have shown that this drug also inhibits COVID-19 viral replication.
MRKPPL’s anti-viral drug has a major role in perturbing the proton motive force (PMF) of the viral cytoplasmic membrane. The product of cellular respiration, PMF, describes the electrochemical potential at the cytoplasmic membrane that is composed of an electrical potential (Δ ψ, negative inside) and a proton gradient (Δ pH, acidic outside). This electrochemical potential crucially underpins energy production so that viral cells work to maintain a constant PMF. Agents that selectively perturb either Δ ψ or Δ pH are known to prompt a compensatory increase in the other component in order to maintain PMF. This anti-viral ligand targets the Δ pH component of PMF. Beta- coronavirus being neutrophilic is most susceptible to the Δ pH component of PMF and leads to sharp reduction in half-life of the virus and aggregation of the S-spikes when reacted with this anti-viral agent.
A crucial step for virus entry into cells is the fusion of its envelope with the membrane of the host cells. To this end, the S proteins of the coronavirus envelope undergo a conformational change that is triggered either upon interaction with the specific cell receptor or by an optimal pH range. Ammonium chloride (at 20 mM) has been described as a non-specific inhibitor of viral replication in vitro, targeting viral uncoating and, at 50 mM, ammonium chloride inhibited cell entry of both SARSCoV and SARS-CoV-2. Chloroquine was also observed to reduce infection of L cells by mouse hepatitis virus 3. Unfortunately, none of these have proven to be effective in treating the Coronavirus and more specifically SARS-CoV-2.
